Introduction
Cocoa powder used in industrial food manufacturing must perform predictably across different applications — chocolate coatings, biscuits, fillings, dairy drinks, and instant beverage powders. One of the most decisive factors influencing this performance is pH level, which is determined during the alkalization stage, also known as the Dutch process.
Alkalization is often described simply as a method to darken cocoa color or soften flavor. In reality, it is a controlled chemical adjustment that stabilizes how cocoa behaves inside complex food systems. The process regulates acidity, dispersion, viscosity interaction, and color development during heating.
Without controlled pH, cocoa powder becomes a variable input. With controlled pH, it becomes a functional industrial ingredient.

alkalized cocoa powder pH control
Natural Cocoa Acidity
Fresh cocoa solids naturally contain organic acids formed during fermentation and roasting. These acids influence taste sharpness, hydration speed, and interaction with fats and proteins.
Typical natural cocoa powder has a pH between 5.0 and 5.8.
At this acidity level:
- dispersion in liquids is slower
- chocolate viscosity increases unpredictably
- dairy protein interaction becomes unstable
- baked color development becomes inconsistent
While natural cocoa is suitable for certain recipes, large-scale manufacturing environments often require more controlled behavior.
Purpose of Alkalization
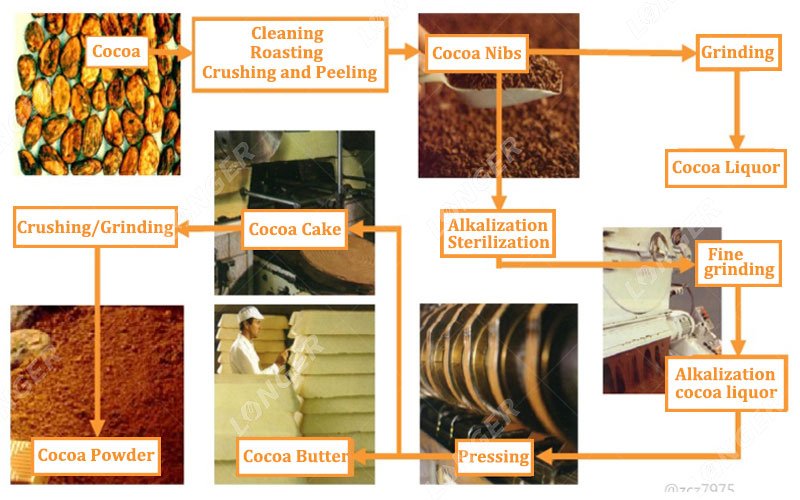
The Dutch process adjusts cocoa pH by treating cocoa cake or nibs with controlled alkaline solutions under regulated temperature and moisture conditions.
The objective is not simply to neutralize acidity but to stabilize functionality.
Proper alkalization creates:
- balanced flavor perception
- improved wettability in mixing
- predictable flow properties
- uniform color formation
- compatibility with fats and proteins
The result is a cocoa powder that integrates consistently into production lines rather than requiring continuous adjustment.
pH Ranges and Industrial Behavior
Different alkalization levels produce different functional profiles.
Each pH range corresponds to specific industrial applications.
Light Alkalized (pH 6.0 – 6.8)
Used where mild color and moderate cocoa taste are required.
Characteristics:
- improved dispersion vs natural cocoa
- controlled bitterness
- suitable for milk beverages and flavored dairy
Medium Alkalized (pH 6.8 – 7.4)
A common industrial range for bakery and compound chocolate.
Characteristics:
- stable baking color
- balanced flavor
- predictable mixing behavior
- good compatibility with sugar and vegetable fats
Heavy Alkalized (pH 7.5 – 8.2)
Used for dark biscuit, wafer cream, and black cocoa applications.
Characteristics:
- deep color stability after heating
- strong visual uniformity
- reduced acidity impact on dough structure
Each range must remain narrow and repeatable. Even a shift of 0.3 pH units can affect viscosity or baked appearance.
Process Control Parameters
Alkalization requires managing several interdependent variables simultaneously.
Moisture
Water activates the chemical reaction and allows uniform penetration.
Too little → uneven reaction
Too much → over-softening of structure
Temperature
Heat determines reaction speed and color development.
Unstable temperature leads to batch variation.
Alkalizing Agent Concentration
The quantity of alkali defines final pH.
Industrial processing uses calibrated dosing rather than approximate addition.
Reaction Time
Under-processing leaves acidity
Over-processing reduces cocoa character and destabilizes performance
Controlled factories monitor all four parameters continuously to ensure repeatable results across production cycles.
Influence on Chocolate Manufacturing
In chocolate systems, cocoa particles interact with fat and sugar networks.
pH level affects rheology.
- lower pH → thicker chocolate mass
- higher pH → smoother flow
Stable alkalization allows manufacturers to maintain fixed fat ratios without reformulation. When pH varies, conching time and viscosity adjustment become necessary.
Industrial processors therefore require cocoa powder that maintains identical pH from shipment to shipment.
Influence on Bakery Products
During baking, cocoa participates in Maillard reactions and thermal color formation.
Uncontrolled pH causes inconsistent appearance between batches.
Consistent alkalization ensures:
- uniform biscuit color
- predictable spread behavior
- stable wafer sheet tone
Production lines operating at fixed oven settings depend on this stability.
Influence on Dairy and Beverage Systems
Milk proteins are sensitive to acidity.
Cocoa with unstable pH can cause separation or sedimentation in beverages.
Controlled alkalization improves:
- suspension stability
- protein compatibility
- color uniformity in drinks
This is particularly critical for ready-to-drink and instant beverage powders.
Relationship Between pH and Particle Structure
Alkalization also affects the internal structure of cocoa solids.
A properly treated cake fractures more uniformly during milling, creating predictable particle distribution.
This leads to:
- smoother mouthfeel
- consistent hydration speed
- stable bulk density
Thus, pH control supports both chemical and mechanical stability.
- alkalized cocoa powder pH control
- alkalized cocoa powder pH control
- alkalized cocoa powder pH control
- alkalized cocoa powder pH control
Repeatability as the Key Industrial Requirement
For large-scale manufacturers, the exact pH value matters less than its consistency.
Production lines are calibrated once and expected to run without correction.
Therefore, reliable cocoa processors design alkalization systems to operate within narrow tolerance ranges across all batches. Industrial buyers typically evaluate not only specification sheets but also the stability of delivered material over time.
In structured processing environments, the Dutch process is treated as a controlled engineering stage rather than a flavor adjustment step.
Subtle Role of Processing Discipline
When alkalization is managed as a standardized operation, cocoa powder behaves predictably in multiple applications. The ingredient becomes compatible with automated mixing, dosing, and thermal treatment systems.
Manufacturers working with consistent material often notice reduced formulation adjustments and stable product appearance across production cycles. This stability originates not from post-production sorting but from precise control during processing.
In professional cocoa processing operations, maintaining repeatable pH behavior is considered one of the core indicators of production maturity.
Conclusion
Alkalization is the central transformation that converts cocoa powder from a naturally acidic material into a controlled industrial ingredient. By regulating pH, the Dutch process stabilizes flavor perception, dispersion, viscosity interaction, and thermal color development.
The success of cocoa powder in chocolate, bakery, and beverage manufacturing depends less on the existence of alkalization and more on the precision of its control. Stable pH values enable consistent processing conditions, reduce downtime, and preserve product uniformity.
In modern food production, the Dutch process is not merely a treatment — it is a technical discipline that ensures cocoa powder behaves as a reliable component within industrial formulations.